Ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's oceans, caused by the uptake of carbon dioxide (CO2) from the atmosphere Seawater is slightly basic (meaning pH > 7), and ocean acidification involves a shift towards pH-neutral conditions rather than a transition to acidic conditions (pH < 7) . An estimated 30–40% of the carbon dioxide from human activity released into the atmosphere dissolves into oceans, rivers and lakes. Some of it reacts with the water to form carbonic acid.
Someof the resulting carbonic acid molecules dissociate into a bicarbonate ion and a hydrogen ion, thus increasing ocean acidity (H+ ion concentration). Between 1751 and 1996, surface ocean pH is estimated to have decreased from approximately 8.25 to 8.14, representing an increase of almost 30% in H+ ion concentration in the world's oceans. Earth System Models project that, by around 2008, ocean acidity exceeded historical analogues and, in combination with other ocean biogeochemical changes, could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean beginning as early as 2100.

2 between the 1700s and the 1990s, from the Global Ocean Data Analysis Project (GLODAP) and the World Ocean Atlas

2 on transect line 5 off Pt. St. George, California. The potential density surfaces are superimposed on the temperature section. The 26.2 potential density surface delineates the location of the first instance in which the undersaturated water is upwelled from depths of 150 to 200 m onto the shelf and outcropping at the surface near the coast. The red dots represent sample locations.[1]
Increasing acidity is thought to have a range of potentially harmful consequences for marine organisms such as depressing metabolic rates and immune responses in some organisms and causing coral bleaching. By increasing the presence of free hydrogen ions, the additional carbonic acid that forms in the oceans ultimately results in the conversion of carbonate ions into bicarbonate ions. Ocean alkalinity (roughly equal to [HCO3−] + 2[CO32−]) is not changed by the process, or may increase over long time periods due to carbonatedissolution. This net decrease in the amount of carbonateions available may make it more difficult for marine calcifying organisms, such as coral and some plankton, to form biogeniccalcium carbonate, and such structures become vulnerable to dissolution. Ongoing acidification of the oceans may threaten future food chains linked with the oceans. As members of the InterAcademy Panel, 105 science academieshave issued a statement on ocean acidification recommending that by 2050, global CO
2 emissions be reduced by at least 50% compared to the 1990 level.
Latest research challenges the potential negative impact of end-of-century ocean acidification level on the coral fish behavior and suggests that the effect could be negligible. Controversially, laboratory experiments in the controlled environment showed CO
2 induced growth of the phytoplankton species. Field study of the coral reef in Queensland and Western Australia from 2007 to 2012 argues that corals are more resistant to the environmental pH changes than previously thought, due to internal homeostasis regulation; this makes thermal change, rather than acidification, the main factor for coral reef vulnerability due to global warming.
While ongoing ocean acidification is at least partially anthropogenic in origin, it has occurred previously in Earth's history, and the resulting ecological collapse in the oceans had long-lasting effects for global carbon cycling and climate. The most notable example is the Paleocene-Eocene Thermal Maximum (PETM), which occurred approximately 56 million years ago when massive amounts of carbon entered the ocean and atmosphere, and led to the dissolution of carbonate sediments in all ocean basins.
Ocean acidification has been compared to anthropogenic climate change and called the "evil twin of global warming" and "the other CO
2 problem". Freshwater bodies also appear to be acidifying, although this is a more complex and less obvious phenomenon. To ensure that ocean acidification is minimized the UN sustainable development goals addresses ocean conservation in sustainable development goal 14 that ensures oceans are conserved and sustainably used.
Issue's of ocean acidification ::-
- Carbon cycle
- Acidification
- Calcification
- Possible impacts
- Possible responses
- Ocean acidification and mass extinction events in the geologic past
- Gallery
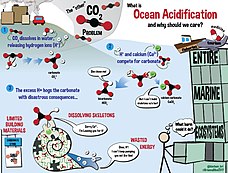
Oceans absorb a substantial proportion of the CO2 emitted into the atmosphere by human activities, with potentially negative effects on shell-forming organisms
What is ocean acidification ?
Human activities release CO2 into the atmosphere, which leads to atmospheric warming and climate change, as explained in Causes of climate change. Around a third to a half of the CO2released by human activities is absorbed into the oceans. While this helps to reduce the rate of atmospheric warming and climate change, it also has a direct, chemical effect on seawater, which we call ocean acidification (Figure 1).
T2I3_Figure-1.gif
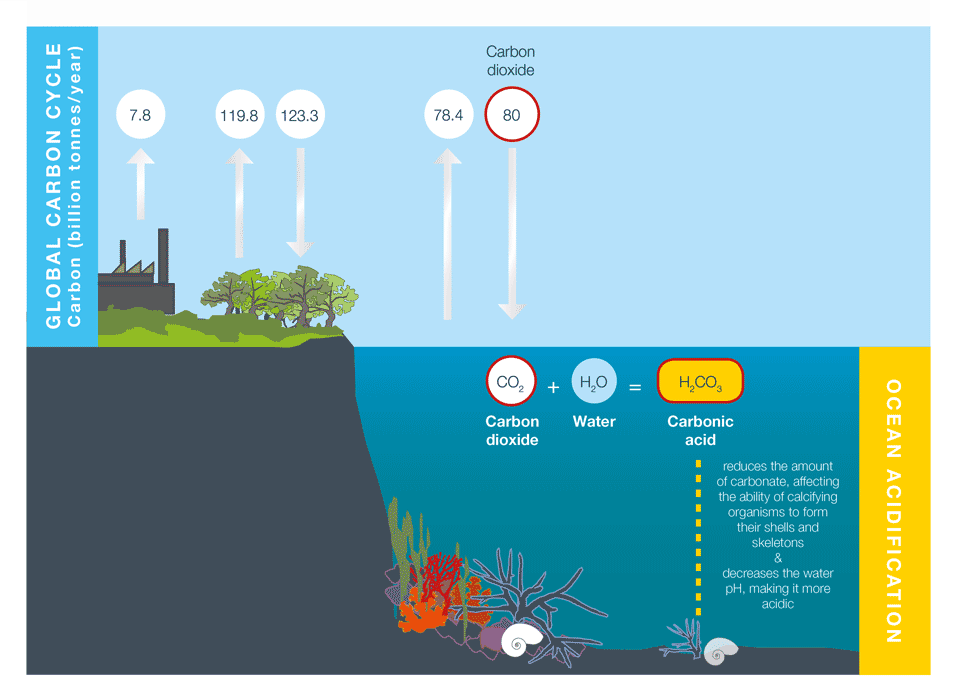
Box 1: What is pH?
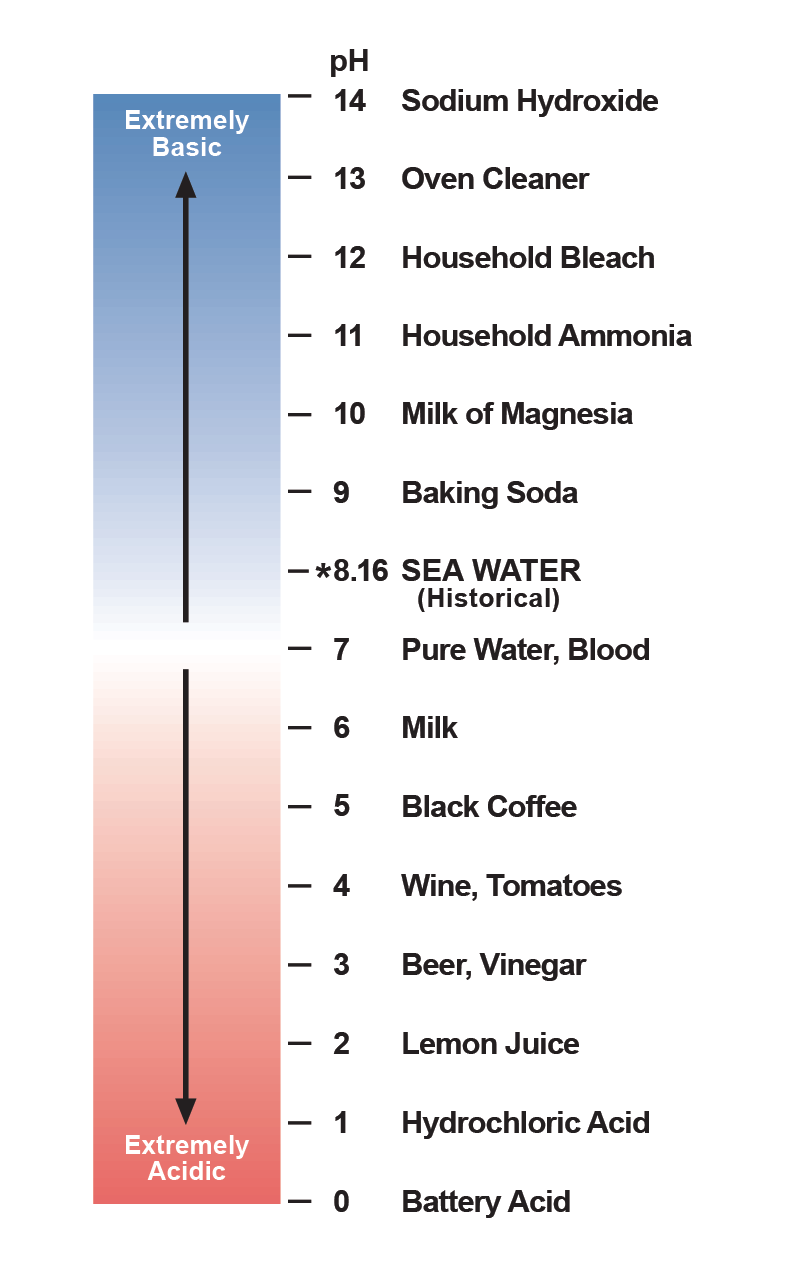
Ocean acidification is often expressed in terms of the pH of seawater. pH is a measure of acidity or alkalinity. A pH below 7 is considered acidic, and a pH greater than 7 is considered alkaline, or basic.
Average ocean water pH is currently 8.1. The pH scale is logarithmic, so a one point change on the scale means a tenfold change in concentration.
T2I3_Figure B1.png
the ob
ceanwhat sbsorb a substantial poportion of the CO2emitted into the atmosphere by human activities, with potentially negative effects on shell-forming organisms.
- Increasing CO2 in the amosphere due to human activities not only affects the climate; it also has direct, chemical effects on ocean waters.
- The oceans have absorbed between a third and a half of the CO2 humans have released into the atmosphere since about 1850. This has slowed the rate of climate change.
- When CO2 dissolves ir, the water becomes more acidic. The acidity of the oceans has increased by 26 % since about 1850, a rate of change roughly 10 times faster than any time in the last 55 million years.
- Associated chemical reactions can make it difficult for marine calcifying organisms, such as coral and some plankton, to form shells and skeletons, and existing shells become vulnerable to dissolution.
- The extent to which calcifying organisms are already being affected by acidification is unclear, as this is a very new area of study. Limited evidence suggests that some organisms are more sensitive than others.
- The rate at which acidification occurs is a determining factor in the extent to which calcifying organisms will be able to adapt.
- The impacts of acidification will extend up the food chain to affect economic activities such as fisheries, aquaculture and tourism. Wherever there are marine calcifying organisms, there are risks from ocean acidification.
Oceans bsorb a substantial proportion of the CO2emitted into the atmosphere by human activities, with potentially negative effects on shell-forming organisms.
- Increasing CO2 in the atmosphere due to human activities not only affects the climate; it also has direct, chemical effects on ocean waters.
- The oceans have absorbed between a third and a half of the CO2 humans have released into the atmosphere since about 1850. This has slowed the rate of climate change.
- When CO2 dissolves in seawater, the water becomes more acidic. The acidity of the oceans has increased by 26 % since about 1850, a rate of change roughly 10 times faster than any time in the last 55 million years.
- Associated chemical reactions can make it difficult for marine calcifying organisms, such as coral and some plankton, to form shells and skeletons, and existing shells become vulnerable to dissolution.
- The extent to which calcifying organisms are already being affected by acidification is unclear, as this is a very new area of study. Limited evidence suggests that some organisms are more sensitive than others.
- The rate at which acidification occurs is a determining factor in the extent to which calcifying organisms will be able to adapt.
- The impacts of acidification will extend up the food chain to affect economic activities such as fisheries, aquaculture and tourism. Wherever there are marine calcifying


Beautiful........
ReplyDelete